A two-component therapy for adults with late-onset Pompe disease (LOPD) weighing 88 lbs or more who are not improving on their current enzyme replacement therapy (ERT).

Pombiliti+Opfoldatreatment every 2 weeks
How to switch from another treatment
If you and your doctor have decided to switch to POMBILITI + OPFOLDA, you can start POMBILITI + OPFOLDA on your next scheduled treatment day (2 weeks after your last ERT dose).
Treatment day at a glance
Like other LOPD treatments, POMBILITI + OPFOLDA therapy is given every 2 weeks. For OPFOLDA to become optimally absorbed and functional inside your bloodstream, it must be taken while you are fasting.
POMBILITI must be taken in combination with OPFOLDA. Because POMBILITI + OPFOLDA were designed to work exclusively with each other, they must be taken at the right time under the right circumstances. Neither should be taken with another Pompe treatment.

The typical treatment day should look like this:
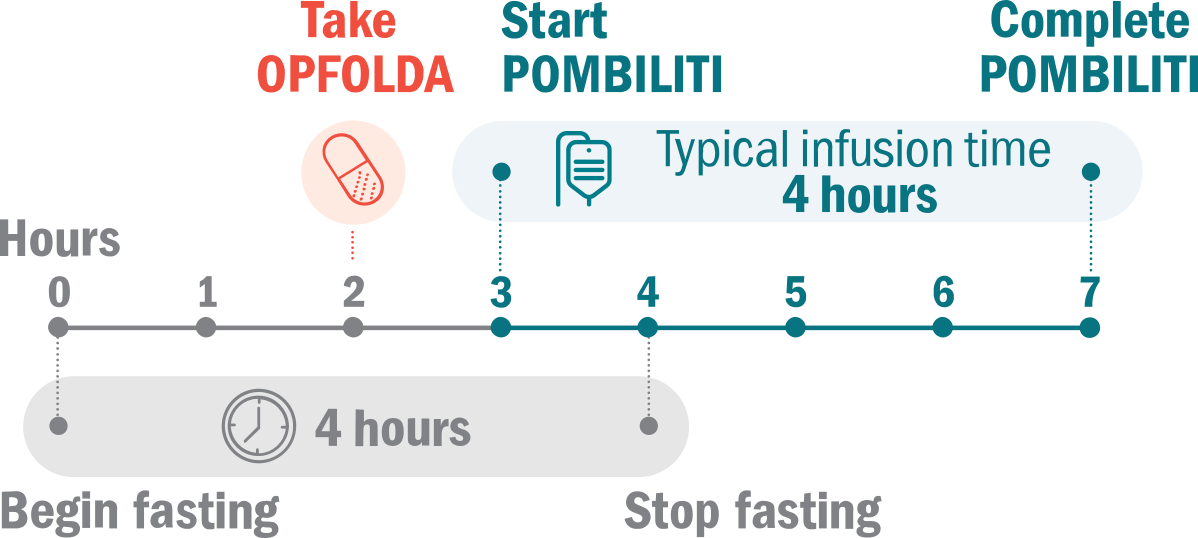
2 hours before taking OPFOLDA:
- Avoid all food and most beverages, to ensure OPFOLDA will be able to bind to POMBILITI in your bloodstream
- It’s okay to drink certain fluids throughout your treatment day
- You may have unsweetened beverages such as water, tea, or coffee with no cream, sugar, or sweeteners
1 hour before your POMBILITI infusion:
- Take OPFOLDA exactly as directed by your healthcare provider
- Swallow OPFOLDA capsules whole only with unsweetened beverages, including water, tea, or coffee with no cream, sugar, or sweeteners
2 hours after taking OPFOLDA:
- You are free to resume normal eating and drinking
Get support options
Find support and other resources that can help you start and stay on track with treatment.
Important Safety Information
POMBILITI in combination with OPFOLDA may cause serious side effects, including:
- Hypersensitivity reactions (including anaphylaxis): Severe and potentially life-threatening allergic-type reactions related to the infusion may occur during and after POMBILITI in combination with OPFOLDA treatment. Your doctor will inform you of the signs and symptoms of hypersensitivity reactions which may include: difficulty breathing or swallowing, rash or hives, low blood pressure, swelling of lips, tongue, throat or face. Seek medical care immediately should signs and symptoms occur. If a severe reaction occurs, your doctor may decide to immediately discontinue the infusion and provide medical care. Appropriate medical support measures may be administered, and you may require close observation during and after POMBILITI infusion.
What are POMBILITI and OPFOLDA?
POMBILITI and OPFOLDA are prescription medicines used in combination for the treatment of adults with late-onset Pompe disease weighing 88 pounds (40 kg) or more and who are not improving on their current enzyme replacement therapy (ERT).
It is not known if POMBILITI in combination with OPFOLDA is safe and effective in children with late-onset Pompe disease.
Important safety information
POMBILITI in combination with OPFOLDA may cause serious side effects, including:
- Hypersensitivity reactions (including anaphylaxis): Severe and potentially life-threatening allergic-type reactions related to the infusion have been reported during and after POMBILITI in combination with OPFOLDA treatment. Your doctor will inform you of the signs and symptoms of hypersensitivity reactions which may include: difficulty breathing or swallowing; rash or hives; low blood pressure; swelling of lips, tongue, throat, or face. Seek medical care immediately should signs and symptoms occur. If a severe reaction occurs, your doctor may decide to immediately discontinue the infusion and provide medical care. Appropriate medical support measures may be administered, and you may require close observation during and after POMBILITI infusion.
- Infusion-Associated Reactions (IARs): Severe IARs related to the infusion have been reported during or after POMBILITI in combination with OPFOLDA. Your doctor will inform you of the signs and symptoms of hypersensitivity reactions which may include: hives, itching, shortness of breath, flushing, chills, and low blood pressure. Seek medical care immediately should signs and symptoms occur. If severe IARs occur during infusion, your doctor may decide to immediately discontinue the infusion and provide appropriate medical care. If you have an acute underlying illness at the time of POMBILITI infusion you may be at greater risk for IARs. If you have advanced Pompe disease you may have compromised heart and breathing function, which may put you at a higher risk of severe complications from IARs.
- Risk of Acute Cardiorespiratory Failure: If you are likely to develop fluid volume overload or have an acute breathing condition or heart and/or breathing problems that require fluid restriction, you may be at risk of worsening of your heart or breathing status during POMBILITI infusion. Your doctor may decide that close observation during and after POMBILITI administration may be necessary.
Do not take POMBILITI in combination with OPFOLDA if you are pregnant.
Before taking POMBILITI in combination with OPFOLDA, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems
- are pregnant or plan to become pregnant. POMBILITI in combination with OPFOLDA may cause harm to your unborn baby.Females who are able to become pregnant:
- Your healthcare provider will check if you are pregnant before you start treatment with POMBILITI in combination with OPFOLDA.
- You should use effective birth control (contraception) during treatment with POMBILITI in combination with OPFOLDA and for at least 60 days after the last dose.
- Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with POMBILITI in combination with OPFOLDA.
- are breastfeeding or plan to breastfeed. It is not known if OPFOLDA alone or in combination with POMBILITI passes into your breast milk. Do not breastfeed during treatment with POMBILITI in combination with OPFOLDA. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
POMBILITI and OPFOLDA must be taken in combination. POMBILITI in combination with OPFOLDA will be given to you 1 time every other week.
The most common side effects of POMBILITI in combination with OPFOLDA include: headache, diarrhea, tiredness, nausea, stomach area pain, and fever.
POMBILITI in combination with OPFOLDA may cause fertility problems in females and males, which may affect the ability to have children. Talk to your healthcare provider if you have concerns about fertility.
These are not all the possible side effects of POMBILITI and OPFOLDA. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
What are POMBILITI and OPFOLDA?
POMBILITI and OPFOLDA are prescription medicines used in combination for the treatment of adults with late-onset Pompe disease weighing 88 pounds (40 kg) or more and who are not improving on their current enzyme replacement therapy (ERT).
It is not known if POMBILITI in combination with OPFOLDA is safe and effective in children with late-onset Pompe disease.
Please see full Prescribing Information, including BOXED WARNING, for POMBILITI and full Prescribing Information and Patient Information for OPFOLDA.

